Multivariant synthesis, crystal structures and properties of four nickel coordination polymers based on flexible ligands†
Abstract
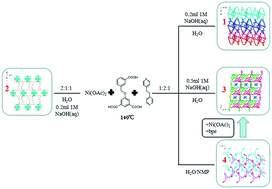
Four new Ni(II) coordination polymers, namely, [Ni(HL)(bpe)(H2O)2]n·nH2O (1), [Ni2(L)(bpe)(μ3-OH)(H2O)]n·3nH2O (2), [Ni(L)(bpe)(H2O)]n·[Ni0.5(bpe)0.5(H2O)2]n·nH2O (3) and [Ni(HL)(bpe)(H2O)]n·3nH2O (4) based on flexible ligands 5-(3-carboxybenzyloxy)-isophthalic acid (H3L) and 1,2-bis(4-pyridyl)ethane (bpe) have been successfully synthesized under different conditions. Compounds 1 and 4 present two-dimensional sql layer structures, while compound 2 exhibits a three-dimensional structure based on a tetranuclear Ni(II) cluster with (3,8)-connected tfz-d topology, and compound 3 consists of a 2D sql anionic layer and 1D linear cationic chains, which are further entangled to give an interesting 2D + 1D → 3D poly-pseudorotaxane framework. To determine the relationship between these diverse structures and external synthetic influence factors, we systematically change the molar ratios of the reactants, solvent and pH and study their effects. Furthermore, the structural transformation from compound 4 to 3 has also been investigated.


 Please wait while we load your content...
Please wait while we load your content...