Crystallization and structural properties of a family of isotopological 3D-networks: the case of a 4,4′-bipy ligand–M2+ triflate system†
Abstract
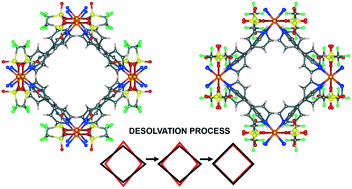
Flexible, stimuli-responsive metal–organic frameworks (MOFs) are very attractive as functional materials due to their wealth of tuneable structural and physical properties. Such compounds are commonly investigated by powder X-ray diffraction (PXRD) or spectroscopy techniques, as in most cases single-crystal X-ray diffraction experiments are not suitable due to process-induced twinning and loss of crystallinity. We re-synthesized a Cu-based flexible porous 3D 2-fold interpenetrated coordination polymer (CUT-1) that bears two kinds of square microporous channels to allocate guest solvent molecules that are lost spontaneously. By crystallizing CUT-1 in a gel medium we were able to increase the mechanical stability and resistance to dehydration of the crystalline samples making it possible to characterize the solvent desorption process at various stages by time-resolved single-crystal X-ray diffraction. The possibility of using other metal ions, such as Cd(II) and Fe(II), to produce a coordination network isostructural with the copper one was also explored in order to highlight the importance of the chemical nature of the metal centre in governing the final structure of the equilibrium product.


 Please wait while we load your content...
Please wait while we load your content...