Liquid phase deposition of iron phosphate thin films†
Abstract
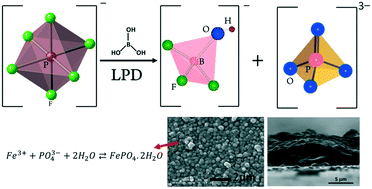
Liquid phase deposition (LPD) has been applied for the synthesis of iron phosphate thin films as an example of an effective methodology for the thin film growth of other polyanionic compounds. The prepared films were highly uniform, free of cracks, pure and successfully applied to cover various substrates such as soda-lime glass and stainless steel. The thickness of these films can be easily tailored in the range of 80 nm to 2.7 μm by the deposition time. The chemical equilibria for the hydrolysis of [PF6]− species and the formation of iron phosphate were studied by 19F-NMR and 31P-NMR spectroscopy to get insight into the formation mechanism of iron phosphate thin films. Hydrolysis of [PF6]− occurs very slowly and a longer induction period favors the hydrolysis. The as-prepared FePO4·2H2O films on conductive stainless steel substrates are shown to be electrochemically active for the intercalation of Li+ ions. Two polymorphs of FePO4 with crystal structures identical to hexagonal tridymite AlPO4 and monoclinic FeAsO4 were identified upon heat treatment of the as-prepared powders.


 Please wait while we load your content...
Please wait while we load your content...