Stacking of cyclopentadienyl organometallic sandwich and half-sandwich compounds. Strong interactions of sandwiches at large offsets†
Abstract
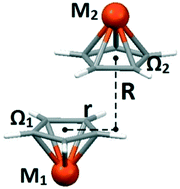
Stacking interactions of organometallic sandwich and half-sandwich compounds with cyclopentadienyl (Cp) were studied by searching and observing the crystal structures in the Cambridge Structural Database and performing density functional calculations. The strongest calculated interactions are at an offset of 1.5 Å with energies for sandwich and half-sandwich dimers of −3.37 and −2.87 kcal mol−1, respectively, somewhat stronger than the stacking interaction between two benzene molecules, −2.73 kcal mol−1. At large offsets of 5.0 Å, 74% of the strongest energy is preserved for the sandwich dimer and only 29% for the half-sandwich dimer. In crystal structures, for sandwich compounds, the stacking at large offsets is dominant (73%), since the interaction at large offsets is relatively strong, and the geometries enable additional simultaneous interactions with Cp faces. The stacking at large offsets between half-sandwich compounds is less dominant, since the interaction is weaker. However, Cp half-sandwich compounds stack at large offsets unexpectedly often (almost 60%), since the branching of their other ligands in the compound favors more simultaneous interactions with Cp faces. Strong interaction at large offsets for sandwich compounds is the consequence of favorable electrostatic interaction, which is not the feature of stacking between half-sandwich compounds.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...