Crystallisation of sodium dodecyl sulfate–water micellar solutions with structurally similar additives: counterion variation†
Abstract
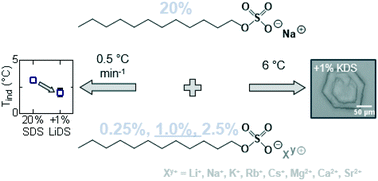
The effects of a series of structurally similar sodium dodecyl sulfate (SDS) additives on the crystallisation of SDS–water micellar solutions were investigated using a combination of differential scanning calorimetry, dynamic light scattering, optical microscopy and inductively coupled plasma optical emission spectroscopy. Seven different counterions were chosen from groups 1 and 2 of the periodic table to replace the sodium on SDS: LDS, (SDS), KDS, RbDS, CsDS, Mg(DS)2, Ca(DS)2 and Sr(DS)2. Two representative temperature profiles were employed – linear cooling ramps at rate of 0.5 °C min−1 to determine near-equilibrium kinetics and transitions and isothermal holds at 6 °C to elucidate morphological changes. Crystallisation of the reference solution 20% SDS–H2O with 0.25, 1.0 and 2.5% additive was generally promoted or inhibited even at the lowest concentrations. Melting points however remained largely unchanged, suggesting that the additives predominantly had a kinetic rather than thermodynamic effect. ICP-OES measurements for the solutions containing 1% additive indicated that most of the additives were integrated into the SDS crystals which was reflected by morphological changes, including the formation of hexagonal and oval shaped crystals. Our results both quantify and provide a morphological insight into the effect of a series of additives on the crystallisation of micellar SDS solutions, which can readily form due to preferential Na exchange.


 Please wait while we load your content...
Please wait while we load your content...