A low-temperature synthesis method for AnO2 nanocrystals (An = Th, U, Np, and Pu) and associate solid solutions
Abstract
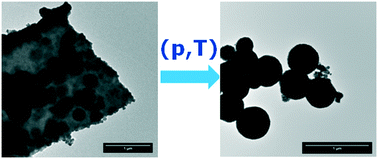
Production of actinide oxide powder via dry thermal decomposition of corresponding oxalates is currently carried out on the industrial scale at temperatures exceeding 500 °C. Although it is simple, this method presents some disadvantages such as high decomposition temperature with a direct effect on the surface area, pre-organised morphology of the nanoparticles affecting the sintering behaviour, etc. We have recently proposed the decomposition of AnIV-oxalates under hot compressed water conditions as a straightforward way to produce reactive actinide oxide nanocrystals. This method could be easily applied at low temperatures (95–250 °C) in order to generate highly crystalline nano-AnO2. We present here the formation conditions of AnO2 (An = Th, U, Np, and Pu) and some associated solid solutions, their stability, and grain growth during thermal treatment. The involvement of water molecules in the mechanism of the oxalate decomposition under the hot compressed water conditions has been demonstrated by an isotopic exchange reaction during the thermal treatment of the hydrated oxalate in H2[17O] through MAS-NMR and Raman techniques.


 Please wait while we load your content...
Please wait while we load your content...