Dimesitylboryl-functionalised cyanostilbene derivatives of phenothiazine: distinctive polymorphism-dependent emission and mechanofluorochromism†
Abstract
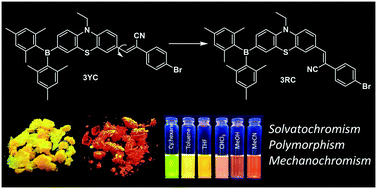
Organic π-conjugated compounds showing polymorphic and mechanofluorochromic (MFC) behaviors are promising candidates for many potential applications in materials chemistry. Herein, we report two distinct molecular designs based on phenothiazine as a donor and triarylborane and cyanostilbene as acceptors, for example, an acceptor–donor–acceptor system (A–D–A; compound 3) and donor–acceptor (D–A; compound 4) moieties, in which 3 exhibits polymorphism with two distinct emissions (yellow and red). The single crystal X-ray diffraction analyses of 3 clearly show two conformational isomers. The color of the emission can be altered by a grinding or fuming process.


 Please wait while we load your content...
Please wait while we load your content...