Preparation of a pure ZIF-67 membrane by self-conversion of cobalt carbonate hydroxide nanowires for H2 separation†
Abstract
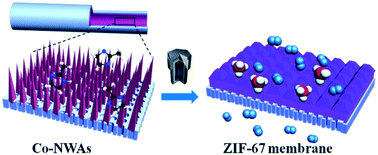
The fabrication of integrated pure Co-based ZIF membranes on porous tubular substrates is extremely challenging. This work demonstrates the preparation of a continuous pure ZIF-67 tubular membrane by direct transformation of carbonate hydroxide nanowire arrays (Co-NWAs) in a 2-methylimidazole (Hmim) aqueous solution. This strategy included first growth of Co-NWAs on a porous ceramic tube and then self-conversion of the Co-NWAs to a continuous ZIF-67 membrane in the Hmim aqueous solution. The effects of the synthesis parameters (solvent, temperature and concentration) on the formation of the pure ZIF-67 membrane were investigated in detail. It was found that the balance between the dissolution rate of the Co-NWAs and the coordination rate of Co2+ produced with Hmim is significant in the transformation process. The obtained ZIF-67 membrane with 1.7 μm thickness exhibits a high H2 permeance of 5.59 × 10−7 mol m−2 s−1 Pa−1, and the ideal separation factors for H2/N2 and H2/CH4 are 14.7 and 15.3, respectively. Moreover, the ZIF-67 membrane demonstrates excellent stability since the Co-NWAs act as strong linkers between the membrane and the substrate. This self-conversion strategy can also be extended to the synthesis of other Co-based MOF membranes.


 Please wait while we load your content...
Please wait while we load your content...