Coordination ability of amino acid hydrazide ligands and their influence on magnetic properties in copper(ii) coordination polymers†
Abstract
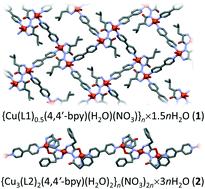
Newly synthesized amino acid hydrazide ligands (L1 = N,N′-di-L-leucine hydrazide; L2 = N,N′-di-L-phenylalanine hydrazide) have shown excellent reactivity and stability, as well as extraordinary coordination ability. Reactions of L1/L2 with copper(II) ions and 4,4′-bipyridine (4,4′-bpy) as a secondary spacer yielded new coordination polymers {Cu(L1)0.5(4,4′-bpy)(H2O)(NO3)}n·1.5nH2O (1) and {Cu3(L2)2(4,4′-bpy)(H2O)2}n(NO3)2n·3nH2O (2). UV/vis spectroscopy together with structural analysis showed that the deprotonated ligands L12−/L22− with six available donor atoms per ligand have the ability for strong chelation to copper(II) ions, acting as bis-bidentate (L12−) and bridging bi + tridentate (L22−) ligands. Compound 1 contains copper(II) ions simultaneously bridged with short hydrazide bridges (4.725 Å) and long 4,4′-bpy spacers (11.144 Å) forming a two-dimensional coordination network. In compound 2, a pair of L22− molecules bridges three copper(II) ions, which are further interconnected with 4,4′-bpy into an overall one-dimensional chain-like structure. Although bulkier, the phenylalanine ligand (L2) was found to be more flexible compared to the leucine ligand (L1), exhibiting additional intramolecular contact: cation–π interaction. In both compounds, the hydrazide (–N–N–) bridge mediates the antiferromagnetic interaction between copper(II) ions. Hydrogen bonds have a strong influence on the crystal packing and physical (magnetic) properties. The reported compounds are first examples of hydrazide-bridged metal centres featuring amino acid-based ligands.


 Please wait while we load your content...
Please wait while we load your content...