Boc-Val-Val-OMe (Aβ39–40) and Boc-Ile-Ala-OMe (Aβ41–42) crystallize in a parallel β-sheet arrangement but generate a different morphology†
Abstract
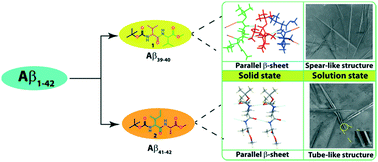
Both N- and C-protected dipeptides, Boc-Val-Val-OMe (1) and Boc-Ile-Ala-OMe (2), bearing sequence homogeneity with the C-terminus of Alzheimer's Aβ39–40 and Aβ41–42, respectively, exhibit intermolecular hydrogen-bonded supramolecular parallel β-sheet structures in crystalline form. But the higher order aggregation of 2 showed a clear supramolecular cross-β-sheet structure unlike that of 1. FESEM images indicated that, while 1 self-assembled into a highly organized two ended spear-like architecture, 2 formed a hollow hexagonal tube-like structure, in a methanol–water solvent mixture. These molecules self-assembled into the ordered structures which found to bind with the amyloid binding dyes thioflavin T (ThT) and Congo red. FT-IR and PXRD also support the formation of the β-sheet structure both in solution and in the solid state. These results may help in understanding amyloidogenesis and design principle of nanostructures.


 Please wait while we load your content...
Please wait while we load your content...