Solid state transformations of different stoichiometric forms of an organic salt formed from 5-sulfosalicylic acid and hexamethylenetetramine upon dehydration and rehydration†
Abstract
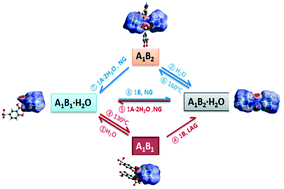
Neat grinding of 5-sulfosalicylic acid (A) and hexamethylenetetramine (B) yields two stoichiometric hydrated salts A1B1·H2O and A1B2·H2O. The hydrated forms A1B1·H2O and A1B2·H2O undergo a reversible solid state interconversion process with anhydrous A1B1 and A1B2 by getting or losing the water molecule. The interconversion between the stoichiometric variations of salts was investigated, in which the dynamic conversion can be obtained by adding one mole of one of the corresponding crystallizing components (A·2H2O or B) upon grinding. Furthermore, the four diverse forms of salts follow transformations reversibly in the solid state. All structural properties have been determined from X-ray diffraction, dynamic vapour sorption, and hot-stage microscopy analysis. The rationalization of the structural changes associated with the transformation has been discussed by combining with Hirshfeld surface analysis.


 Please wait while we load your content...
Please wait while we load your content...