Selective MeCN/EtCN sorption and preferential inclusion of substituted benzenes in a cage structure with arylsulfonamide-armed anthraquinones†
Abstract
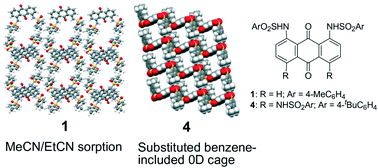
We report a series of crystal structures of arylsulfonamide-armed anthraquinones (AQs) (1–4). The arylsulfonamide-armed AQs formed orthogonal aromatic arrangements between the AQ unit and terminal aryl units due to well-defined intramolecular hydrogen bonding between the carbonyl units of AQs and the amino groups of sulfonamide units. Three disubstituted AQs 1–3 formed fundamental dimer structures, which were stabilized by intermolecular π–π interaction between AQs. Subtle differences in the dimer structures led to different packing structures. Among them, the 1,8-bis(arylsulfonamide) derivative (1) formed solvated crystals of 1·(MeCN), which exhibited reversible and selective MeCN and/or EtCN adsorption–desorption behavior. Tetra(arylsulfonamide) AQ (4) with four bulky substituents on its periphery formed various host–guest molecular crystals of 4·X2 (X = toluene, xylene, trimethylbenzenes, 1,2,3,5-tetramethylbenzene, anisole, and benzonitrile) with a rectangular zero-dimensional cage surrounded by the π-planes of 4.


 Please wait while we load your content...
Please wait while we load your content...