Palladium-catalyzed intramolecular transfer hydrogenation & cycloaddition of p-quinamine-tethered alkylidenecyclopropanes to synthesize perhydroindole scaffolds†
Abstract
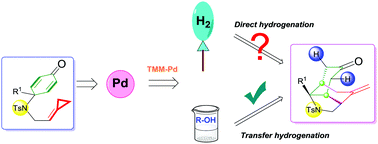
A palladium(0)-catalyzed intramolecular transfer hydrogenation and cycloaddition of p-quinamine-tethered alkylidenecyclopropanes (ACPs) to synthesize perhydroindole scaffolds has been reported in this communication. Mechanistic investigations on the basis of deuterium labeling experiments suggest that the reaction proceeded through an oxidative addition of Pd(0) into the distal bond of the ACP moiety to afford a trimethylenemethane (TMM)–Pd intermediate followed by transfer hydrogenation using alcohol.


 Please wait while we load your content...
Please wait while we load your content...