Carbon–nitrogen bond cleavage of pyridine with two molecular substituted allenoates: access to 2-arylpyrimidin-4(3H)-one†
Abstract
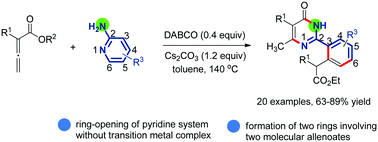
A DABCO-catalyzed annulation reaction of pyridin-2-amine and substituted allenoates has been disclosed. This strategy allows for the ring-opening of a pyridine ring system and the formation of two new rings including a pyrimidinone ring and a benzene ring in an efficient manner.


 Please wait while we load your content...
Please wait while we load your content...