Bioorthogonal release of sulfonamides and mutually orthogonal liberation of two drugs†
Abstract
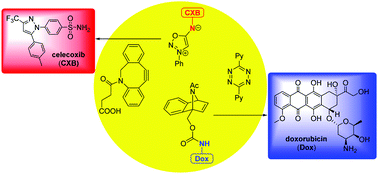
Sulfonamide derivatives have been used in pharmaceutics for decades. Here we report a new approach to release sulfonamides efficiently using a bioorthogonal reaction of sulfonyl sydnonimines and dibenzoazacyclooctyne (DIBAC). The second-order rate constant of the cycloaddition reaction can be up to 0.62 M−1 s−1, and the reactants are highly stable under physiological conditions. Most significantly, we also discovered the mutual orthogonality between the sydnonimine–DIBAC and benzonorbornadiene–tetrazine cycloaddition pairs, which can be used for selective and simultaneous liberation of sulfonamide and primary amine drugs.


 Please wait while we load your content...
Please wait while we load your content...