Facile insertion of ethylene into a group 14 element-carbon bond: effects of the HOMO–LUMO energy gap on reactivity†
Abstract
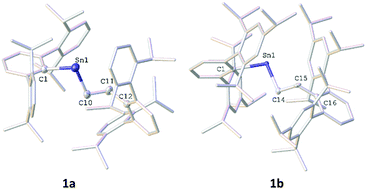
The diarylstannylenes, Sn(AriPr4)2 and Sn(AriPr6)2, (AriPr4 = C6H3-2,6-(C6H3-2,6-iPr2)2, AriPr6 = C6H3-2,6-(C6H2-2,4,6-iPr3)2), undergo a facile migratory insertion reaction with ethylene at 60 °C to afford the alkyl aryl stannylenes AriPr4SnCH2CH2AriPr4 and AriPr6SnCH2CH2AriPr6 which were characterized via1H, 13C and 119Sn NMR, UV-vis and IR spectroscopy, as well as by X-ray crystallography. Quantum mechanical calculations were performed, and two potential mechanisms were identified, with a migratory insertion reaction pathyway being energetically preferred.


 Please wait while we load your content...
Please wait while we load your content...
