Metal free direct C(sp2)–H arylaminations using nitrosoarenes to 2-hydroxy-di(het)aryl amines as multifunctional Aβ-aggregation modulators†
Abstract
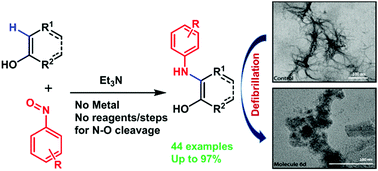
An unprecedented metal free arylamination reaction involving nitrosoarenes as the electrophilic aminating agents is reported. The direct arylamination of a broad range of substrates, such as naphthols, hydroxyquinolines, hydroxyquinones, coumarins and 1,3-cyclohexadienones was achieved under operationally simple and mild conditions without the aid of additional reagents/steps for N–O bond reduction. Interestingly, novel 2-hydroxydiaryl amines were found to act as Aβ-aggregation inhibitors.
- This article is part of the themed collection: New Frontiers in Indian Research


 Please wait while we load your content...
Please wait while we load your content...