Lewis acid catalysis: regioselective hydroboration of alkynes and alkenes promoted by scandium triflate†
Abstract
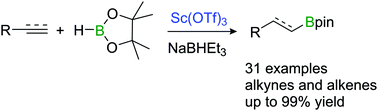
The first commercially available scandium-catalysed selective hydroboration of alkynes and alkenes with HBpin (pin = OC-Me2CMe2O) in the presence of a catalytic amount of NaHBEt3 has been developed. This protocol can be applicable to a wide range of substrates including aromatic, aliphatic with cyclic and acyclic side chains, and heteroaryl systems with broad functional-group compatibility. Mechanistic studies revealed that the reaction occurs in a syn fashion via the σ-bond metathesis between the alkenyl scandium species and HBpin.


 Please wait while we load your content...
Please wait while we load your content...