A catalytic one-step synthesis of peptide thioacids: the synthesis of leuprorelin via iterative peptide–fragment coupling reactions†
Abstract
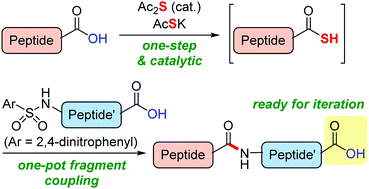
A catalytic one-step synthesis of peptide thioacids was developed. The oxygen–sulfur atom exchange reaction converted the carboxy group at the C-terminus of the peptides into a thiocarboxy group with suppressed epimerization. This method was successfully applied to the synthesis of the peptide drug leuprorelin via an iterative fragment-coupling protocol.


 Please wait while we load your content...
Please wait while we load your content...