An integrated immobilization strategy manipulates dual active centers to boost enantioselective tandem reactions†
Abstract
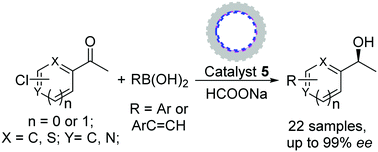
Manipulation of dual active centers via an integrated immobilization strategy can overcome the restriction of homogeneous catalysis in a sequential organic transformation. Herein, by utilizing hollow-shell-structured silica, hydrogen-bonding immobilization via a ship-in-a-bottle synthesis locks a Pd(carbene) center in a nanocage and covalent-bonding immobilization tethers chiral Ru(diamine) centers within the nanochannels, constructing a hetero-bifunctional catalyst. The benefit of this dual center manipulation enables a challenging Suzuki coupling-asymmetric transfer hydrogenation tandem reaction, and the advantage of this process provides various chiral biarylols with enhanced reactivity and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...