Difunctionalization of ketones via gem-bis(boronates) to synthesize quaternary carbon with high selectivity†
Abstract
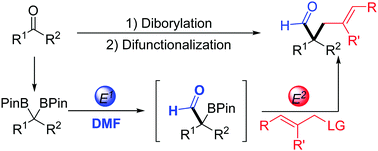
All-carbon quaternary centres are significant and prevalent structural frameworks but their preparation routes are rare and challenging, especially methods with common substrates. Herein, we report a convenient process to construct all-carbon quaternary centres from ketones via the diborylation process and Suzuki–Miyaura cross-coupling reaction. This methodology, which simultaneously introduces two different kinds of electrophilic structures, exhibits a large substrate scope and high functional group tolerance. The reaction products with aldehyde and allylic groups have proved to be versatile synthons to prepare complex molecules crucial for natural product synthesis.


 Please wait while we load your content...
Please wait while we load your content...