Abstract
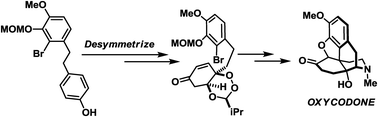
Here we report a total synthesis of the pharmacologically significant morphinan alkaloid, oxycodone. The centerpiece of the developed strategy features the first application of the Rovis desymmetrization of peroxyquinol in target-oriented total synthesis to access an optically active phenanthrene framework shared by the morphinans. A Stork–Ueno radical cyclization under photoredox conditions installed the all-carbon quaternary stereocenter, and a late-stage reductive detosylation with concomitant piperidine formation secured the core structure of the target molecule.
- This article is part of the themed collection: Natural product synthesis


 Please wait while we load your content...
Please wait while we load your content...