Alkylarylation of styrenes via direct C(sp3)–Br/C(sp2)–H functionalization mediated by photoredox and copper cooperative catalysis†
Abstract
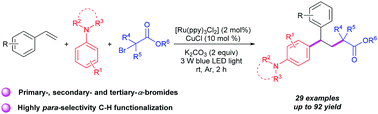
For straightforward access to various substituted 1,1-diarylalkanes a photoredox-catalyzed and copper-promoted 1,2-alkylarylation reaction of styrenes has been developed, which uses α-carbonyl alkyl bromides and N,N-disubstituted anilines as functionalization reagents. In this radical difunctionalization reaction, α-carbonyl alkyl bromides, including primary-, secondary- and tertiary-α-bromoalkyl ketone esters, malonic esters and cycloalkane were transformed to the corresponding 1,1-diarylalkanes in moderate to good yields at room temperature. Notably, this transformation provided a new route for the C–H alkylation of N,N-disubstituted anilines with high para-selectivity beyond the typical Friedel–Crafts alkylation.


 Please wait while we load your content...
Please wait while we load your content...