Catalytic enantioselective ene-type reactions of vinylogous hydrazone: construction of α-methylene-γ-butyrolactone derivatives†
Abstract
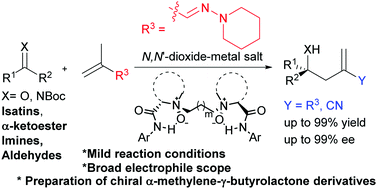
The catalytic asymmetric ene-type reactions of vinylogous hydrazone were accomplished by using chiral N,N′-dioxide–metal salt complexes as catalysts. A wide range of electrophiles, including isatins, α-ketoester, imines, and aldehydes reacted with (E)-2-methyl-N-(piperidin-1-yl)prop-2-en-1-imine efficiently, affording the corresponding homoallylic alcohols and amines in high yields (up to 99%) with excellent ee values (up to 99%). The methodology provided a convenient way to synthesize bioactive chiral α-methylene-γ-butyrolactone derivatives.


 Please wait while we load your content...
Please wait while we load your content...