Asymmetric synthesis of Rauhut–Currier-type esters via Mukaiyama–Michael reaction to acylphosphonates under bifunctional catalysis†
Abstract
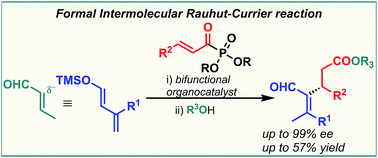
A highly enantioselective organocatalytic Mukaiyama–Michael reaction of silyloxy dienes and α,β-unsaturated acyl phosphonates under bifunctional organocatalysis is presented. The new reactivity triggered by the catalyst conducted to Rauhut–Currier type esters, via a formal conjugate addition to α,β-unsaturated esters. This protocol proceeds under mild conditions with complete regioselectivity and excellent enantiocontrol.


 Please wait while we load your content...
Please wait while we load your content...