Development of efficient palladium catalysts for alkoxycarbonylation of alkenes†
Abstract
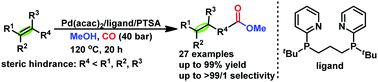
Herein, we report a general and efficient Pd-catalysed alkoxycarbonylation of sterically hindered and demanding olefins including a variety of tri-, tetra-substituted and 1,1-disubstituted alkenes. In the presence of 1,3-bis(tert-butyl(pyridin-2-yl)phosphanyl)propane L3 or 1,4-bis(tert-butyl(pyridin-2-yl)phosphanyl)butane L4 the desired esters are obtained in good yields and selectivities. Similar transformation is obtained using tertiary ether as showcased in the carbonylation of MTBE to the corresponding linear ester in high yield and selectivity.


 Please wait while we load your content...
Please wait while we load your content...