Stereogenic cis-2-substituted-N-acetyl-3-hydroxy-indolines via ruthenium(ii)-catalyzed dynamic kinetic resolution-asymmetric transfer hydrogenation†
Abstract
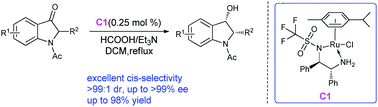
Ruthenium(II)-catalyzed dynamic kinetic resolution-asymmetric transfer hydrogenation of racemic 2-substituted-N-acetyl-3-oxoindolines to cis-2-substituted-N-acetyl-3-hydroxyindolines is reported. Using the homochiral {Ru[TfDPEN](p-cymene)} catalyst with S/C = 400 in a HCO2H/Et3N mixture, up to >99.9% ee and >99 : 1 dr are obtained with high yields (79–98%). This method provides the first example of preparing enantiomerically pure indolines through asymmetric transfer hydrogenation (ATH).


 Please wait while we load your content...
Please wait while we load your content...