Iridium-catalyzed direct asymmetric vinylogous allylic alkylation†
Abstract
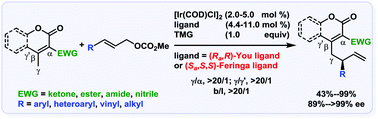
The catalytic asymmetric vinylogous allylic alkylation of α,β-unsaturated lactones (including coumarins) was achieved with excellent regio- and enantioselectivity. Transformations of the product were carried out by means of the versatile terminal olefin and lactone moieties. The synthetic application of the present methodology was showcased by the asymmetric synthesis of an advanced synthetic Merck intermediate toward a new drug candidate.


 Please wait while we load your content...
Please wait while we load your content...