Nucleophilic ring opening of trans-2,3-disubstituted epoxides to β-amino alcohols with catalyst-controlled regioselectivity†
Abstract
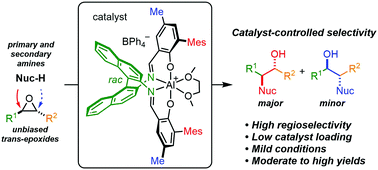
We report the nucleophilic ring opening of unsymmetrical trans-epoxides to β-amino alcohols with catalyst-controlled regioselectivity. This cationic aluminum salen catalyst, which contains bulky mesityl groups in the ortho-position of the phenoxide and a 2,2′-diamino-1,1′-binaphthalene backbone, transforms a variety of epoxides with high regioselectivity using nitrogen-containing nucleophiles. Unlike most reports, in which regioselectivity is substrate controlled, the regioselectivity in this system is catalyst controlled and allows selective nucleophilic ring opening of unbiased trans-epoxides.


 Please wait while we load your content...
Please wait while we load your content...