Nickel(ii)-catalyzed enantioselective α-alkylation of β-ketoamides with phenyliodonium ylide via a radical process†
Abstract
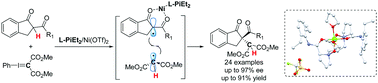
Chiral Lewis acid-promoted enantioselective α-alkylation of β-ketoamides with phenyliodonium ylide was developed. In the presence of a chiral N,N′-dioxide/Ni(II) complex, the corresponding 1,4-dicarbonyl compounds were obtained with good results (up to 91% yield and 97% ee). Control experiments and EPR studies supported a radical process.


 Please wait while we load your content...
Please wait while we load your content...