Expeditious synthesis of multisubstituted indoles via multiple hydrogen transfers†
Abstract
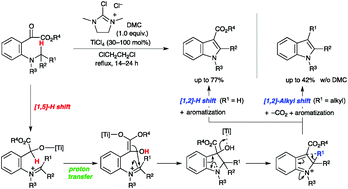
Described herein is the expeditious construction of multi-substituted indole skeletons via multiple hydrogen transfers. When ortho-amino ketoesters were treated with a catalytic amount of TiCl4 in the presence of 1.0 equivalent of dehydrating reagent, three types of hydrogen transfer processes ([1,5]-hydride shift, proton transfer, and [1,2]-hydride shift) occurred to give various 3-alkoxycarbonylindoles. Further study revealed that a [1,2]-alkyl shift instead of a [1,2]-hydride shift proceeded to afford 3-alkylindoles from the substrates with an amino group having tertiary carbons adjacent to a nitrogen atom.


 Please wait while we load your content...
Please wait while we load your content...