Enantioselectively functionalised phenytoin derivatives by auxiliary-directed N to C aryl migration in lithiated α-amino nitriles†
Abstract
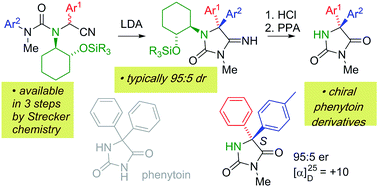
Lithiation of N′-arylureas derived from amino nitriles incorporating a (1R,2R)-2-aminocyclohexanol chiral auxiliary leads to diastereoselective migration of the aryl ring to the position α to the nitrile. The resulting N′-lithiated ureas undergo spontaneous cyclisation to iminohydantoins, which may be hydrolysed to give chiral 5,5-diarylhydantoins related to phenytoin, in enantioenriched form.


 Please wait while we load your content...
Please wait while we load your content...