Synthesis of selenocysteine-containing cyclic peptides via tandem N-to-S acyl migration and intramolecular selenocysteine-mediated native chemical ligation†
Abstract
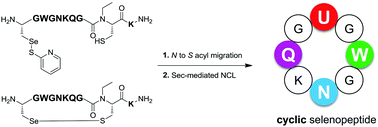
Selenocysteine-containing cyclic 8-mer peptides, which were designed to mimic the plausible catalytic tetrad of glutathione peroxidase, were successfully synthesized in one pot via tandem N-to-S acyl migration of N-alkylcysteine (NAC)-containing selenopeptides and intramolecular selenocysteine-mediated native chemical ligation (Sec-NCL) of the generated thioesters.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...