Does deamidation of islet amyloid polypeptide accelerate amyloid fibril formation?†
Abstract
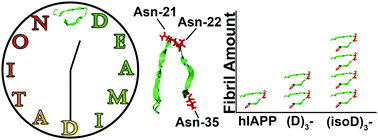
Mass spectrometry has been applied to determine the deamidation sites and the aggregation region of the deamidated human islet amyloid polypeptide (hIAPP). Mutant hIAPP with iso-aspartic residue mutations at possible deamidation sites showed very different fibril formation behaviour, which correlates with the observed deamidation-induced acceleration of hIAPP aggregation.


 Please wait while we load your content...
Please wait while we load your content...