The Strecker reaction coupled to Viedma ripening: a simple route to highly hindered enantiomerically pure amino acids†
Abstract
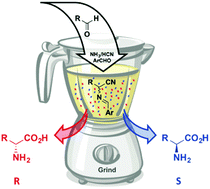
The Strecker reaction is broadly used for the preparation of α-amino acids. However, control of enantioselectivity remains challenging. We here couple the Strecker reaction to Viedma ripening for the absolute asymmetric synthesis of highly sterically hindered α-amino acids. As proof-of-principle, the enantiomerically pure α-amino acids tert-leucine and α-(1-adamantyl)glycine were obtained.


 Please wait while we load your content...
Please wait while we load your content...