Synthesis of multisubstituted pyrroles by nickel-catalyzed arylative cyclizations of N-tosyl alkynamides†
Abstract
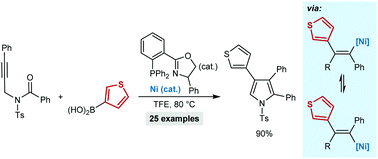
The synthesis of multisubstituted pyrroles by the nickel-catalyzed reaction of N-tosyl alkynamides with arylboronic acids is reported. These reactions are triggered by alkyne arylnickelation, followed by cyclization of the resulting alkenylnickel species onto the amide. The reversible E/Z isomerization of the alkenylnickel species is critical for cyclization. This method was applied to the synthesis of pyrroles that are precursors to BODIPY derivatives and a biologically active compound.


 Please wait while we load your content...
Please wait while we load your content...