Self-assembly of penta-selenopeptides into amyloid fibrils†
Abstract
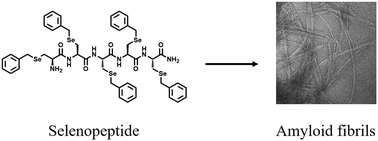
Here, we report the synthesis of a penta-selenopeptide consisting of five benzyl protected selenocysteine residues. This selenopeptide was well characterized by both one- and two-dimensional (D) NMR spectroscopies. We find that the solution conformation is enriched with β-sheet structures, which have a propensity to self-assemble and form amyloid fibrils.
- This article is part of the themed collection: ChemComm Advisory Board 2020


 Please wait while we load your content...
Please wait while we load your content...