Synthesis of acyl fluorides via photocatalytic fluorination of aldehydic C–H bonds†
Abstract
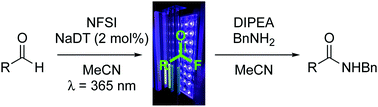
Acyl fluorides are versatile acylating agents owing to their unique stability. Their synthesis, however, can present challenges and is typically accomplished through deoxyfluorination of carboxylic acids. Here, we demonstrate that acyl fluorides can be prepared directly from aldehydes via a C(sp2)–H fluorination reaction involving the inexpensive photocatalyst sodium decatungstate and electrophilic fluorinating agent N-fluorobenzenesulfonimide. This convenient fluorination strategy enables direct conversion of aliphatic and aromatic aldehydes into acylating agents.


 Please wait while we load your content...
Please wait while we load your content...