Direct monitoring of the conformational equilibria of the activation loop in the mitogen-activated protein kinase p38α†
Abstract
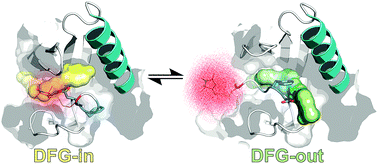
Conformational transitions in protein kinases are crucial for the biological function of these enzymes. Here, we characterize and assess conformational equilibria of the activation loop and the effect of small molecule inhibitors in the MAP kinase p38α. Our work experimentally revealed the existence of a two-state equilibrium for p38α while the addition of inhibitors shifts the equilibrium between these two states.


 Please wait while we load your content...
Please wait while we load your content...