Further enhancement of the clickability of doubly sterically-hindered aryl azides by para-amino substitution†
Abstract
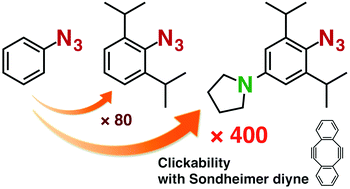
Introduction of an amino group at the para position of doubly sterically-hindered aryl azides significantly enhances the reactivity with cyclooctynes. The distortability of the azido group is synergistically enhanced by the para-electron-donating group and two bulky ortho substituents, which increases the HOMO energy level and provoke the steric inhibition of resonance, respectively.


 Please wait while we load your content...
Please wait while we load your content...