Diversity-oriented synthesis of chromenopyrrolidines from azomethine ylides and 2-hydroxybenzylidene indandiones via base-controlled regiodivergent (3+2) cycloaddition†
Abstract
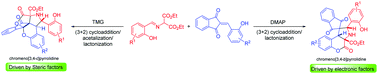
An organobase-directed, regiodivergent 1,3-dipolar cycloaddition of azomethine ylides and 2-hydoxybenzylidene indandiones is reported. The scarcely explored reversal of the nucleophilic site in azomethine ylides has been exploited for their regiodivergent (3+2) cycloaddition, which subsequently resulted in two different cascade processes to generate functionally distinct chromenopyrrolidines in a diversity oriented manner.


 Please wait while we load your content...
Please wait while we load your content...