Neutral and cationic tungsten(vi) fluoride complexes with tertiary phosphine and arsine coordination†
Abstract
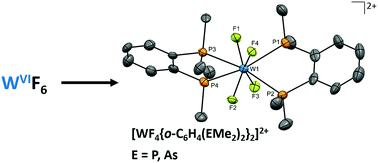
Reaction of WF6 with AsR3 (R = Me or Et) in anhydrous CH2Cl2 at low temperature forms the neutral seven-coordinate, [WF6(AsR3)] (R = Me, Et), the first arsine complexes of WF6, whilst o-C6H4(EMe2)2 (E = P, As) produces [WF4{o-C6H4(EMe2)2}2][WF7]2. Crystal structures show the latter contain dodecahedral cations, and present the highest oxidation state metal fluoride complexes known (and the highest possible for tungsten) with soft neutral phosphine and arsine coordination.
- This article is part of the themed collection: Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...