Access towards enantiopure α,α-difluoromethyl alcohols by means of sulfoxides as traceless chiral auxiliaries†
Abstract
A new methodology to access enantiopure α,α-difluoromethyl alcohols is hereby being described. The strategy relies on the use of an enantiopure aryl α,α-difluoromethyl sulfoxide employed as chiral and removable auxiliary for the stereoselective difluoromethylation of carbonyl derivatives. The obtained α,α-difluoro-β-hydroxysulfoxides displayed unprecedented diastereomeric ratios.
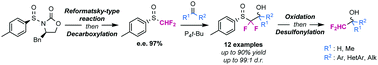
- This article is part of the themed collection: Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...