Hydrogen peroxide production via a redox reaction of N,N′-dimethyl-2,6-diaza-9,10-anthraquinonediium by addition of bisulfite†
Abstract
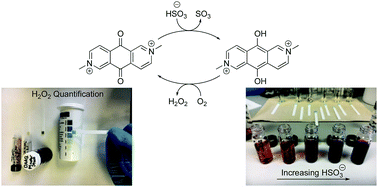
We demonstrate that bisulfite can be used for reduction of a highly electrophilic anthraquinone derivative, N,N′-dimethyl-2,6-diaza-9,10-anthraquinonediium (DAAQ), and subsequent autoxidation generates an equivalent of hydrogen peroxide. The mechanism for DAAQ reduction by bisulfite, DAAQ electrochemistry, and use of a simple test strip assay for H2O2, are described.
- This article is part of the themed collection: Celebrating our 2020 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...