Visible-light photocatalyzed oxidative decarboxylation of oxamic acids: a green route to urethanes and ureas†
Abstract
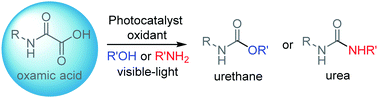
A sustainable metal-free route to urethanes and ureas based on a photocatalyzed oxidative decarboxylation of oxamic acids is described. The reaction includes in situ generation of an isocyanate from the oxamic acid, using an organic dye as a photocatalyst, a hypervalent iodine reagent as an oxidant and a light source, which trigger the free-radical decarboxylation. This protocol successfully avoids the isolation, purification and storage of carcinogenic isocyanates and allows elaboration of urethanes and ureas in a one-pot process from commercially available sources.


 Please wait while we load your content...
Please wait while we load your content...