Chiral Lewis acid-catalyzed enantioselective cyclopropanation and C–H insertion reactions of vinyl ketones with α-diazoesters†
Abstract
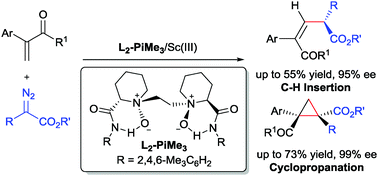
Enantioselective cyclopropanation and C–H insertion reactions of α-substituted vinyl ketones with α-diazoesters have been accomplished using a N,N′-dioxide-scandium(III) complex catalyst. Various tetrasubstituted cyclopropanes and E-enone derivatives bearing a chiral ester substituent were obtained simultaneously with good yields and excellent enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...