The CF3-DAST-induced deacylative trifluoromethylthiolation of cyclic 1,3-diketones/lactams/lactones and its extension to deacylative pentafluorophenylthiolation†
Abstract
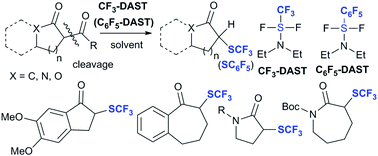
A trifluoromethyl analogue of diethylaminosulfur trifluoride (CF3-DAST)-induced deacylative trifluoromethylthiolation of cyclic 1,3-diketones, lactams, and lactones that provides cyclic α-trifluoromethylthioketones, lactams, and lactones is reported. To the best of our knowledge, this method represents the first example of the trifluoromethylthiolation of lactams. A corresponding deacylative pentafluorophenylthiolation using a pentafluorophenyl analogue of diethylaminosulfur trifluoride (C6F5-DAST) was also attempted.
- This article is part of the themed collection: Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...