Design and synthesis of tailored human caseinolytic protease P inhibitors†
Abstract
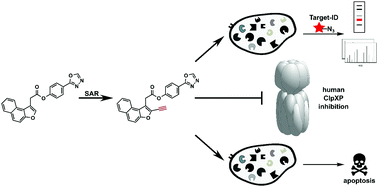
Human caseinolytic protease P (hClpP) is important for degradation of misfolded proteins in the mitochondrial unfolded protein response. We here introduce tailored hClpP inhibitors that utilize a steric discrimination in their core naphthofuran scaffold to selectively address the human enzyme. This novel inhibitor generation exhibited superior activity compared to previously introduced beta-lactones, optimized for bacterial ClpP. Further insights into the bioactivity and binding to cellular targets were obtained via chemical proteomics as well as proliferation- and migration studies in cancer cells.


 Please wait while we load your content...
Please wait while we load your content...