Oximinotrifluoromethylation of unactivated alkenes under ambient conditions†
Abstract
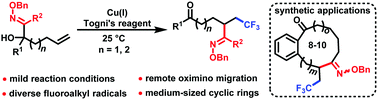
An efficient protocol for oximinotrifluoromethylation of unactivated alkenes was developed via trifluoromethyl radical-induced intramolecular remote oximino migration under mild reaction conditions, providing a wide range of β-trifluoromethylated oximes. Other fluoroalkyl radicals were also applicable for this transformation. This method provided access to synthetically challenging medium-sized ring scaffolds and the 6,7,5-fused lactam skeleton.
- This article is part of the themed collection: Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...