Synthesis of heteroaromatic trifluoromethyl ethers with trifluoromethyl triflate as the source of the trifluoromethoxy group†
Abstract
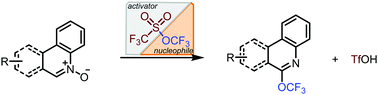
A series of nitrogen-heterocycles have been transformed to the corresponding trifluoromethyl or higher perfluoroalkyl ethers by reaction of the corresponding N-oxides with trifluoromethyl or higher perfluoroalkyl triflate. Trifluoromethyl triflate, which has generally been used as a precursor to [OCF3]−, is used here as a bifunctional reagent to render the heteroarene more electrophilic and to deliver the trifluoromethoxy group. This reagent was easily prepared on a large scale (>100 grams) and is stable in either pure form or as a stock solution. Applications and limitations of this method are reported.


 Please wait while we load your content...
Please wait while we load your content...